Osmosis
Osmosis (/ɒzˈmoʊ.sɪs/) is the movement of solvent (liquid) molecules through a membrane, from one solution to another, which happens without an outside force.
Solvent will move to the side that has a higher concentration of solutes, or dissolved particles — and thus a lower concentration of solvent. This happens because the membrane is selectively permeable: the solvent can pass through, but the solute cannot. Solvent molecules move randomly, and so the concentrations on both sides become more equal.
Osmosis can be made to do work. Osmotic pressure is the external pressure that must be applied so that there is no net movement of solvent across the membrane. Osmotic pressure depends on the molar concentration of the solute.
Osmosis is important in biological (living) systems, as biological membranes are semipermeable. In general, these membranes are impermeable to large molecules such as ions, proteins, and polysaccharides. They are permeable to non-polar or hydrophobic molecules like lipids and small molecules like oxygen, carbon dioxide, nitrogen, and nitric oxide. Permeability depends on solubility, charge, or chemistry, as well as solute size. Water molecules travel through the plasma membrane, vacuole or protoplast by diffusing across the phospholipid bilayer.
Osmosis provides the main way water is gets in and out of cells. The turgor pressure of a cell is largely maintained by osmosis across the cell membrane between the cell interior and its environment.


Hypotonic, isotonic, and hypertonic[change | change source]
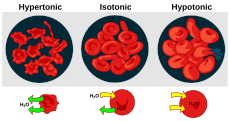
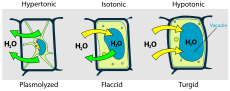
Solutions may have more or less solute per unit of solvent. The one with less is called hypotonic. When the two solutions have equal concentration, they are isotonic. The one with more is hypertonic. When the hypotonic solution is outside the cell, and hypertonic solution inside, the cell becomes swollen and distorted.
Cell membranes[change | change source]
The plasma membrane of a cell is semi-permeable, which means it allows the entry of certain molecules in or out. It lets small molecules pass through, but blocks larger molecules,. The membrane also has ports or gateways which get certain macromolecules through. This is active transport, which uses energy and is selective. It is the outermost covering of the animal cell, made of proteins and lipids. Example: exchange of gases like oxygen and carbon dioxide.
