Chemical reaction


A chemical reaction happens when one or more chemicals are changed into one or more other chemicals. Examples:
- iron and oxygen combining to make rust
- vinegar and baking soda combining to make sodium acetate, carbon dioxide and water
- things burning or exploding
- many reactions that happen inside living things, such as photosynthesis
- electrochemical reactions when discharging or recharging batteries
Some reactions are fast, and others are slow. Some happen at different speeds, depending on temperature or other things. For example, wood does not react with air when it is cold, but if it is made hot enough, it will start to burn. Some reactions give out energy. These are exothermic reactions. In other reactions, energy is taken in. These are endothermic reactions.
Nuclear reactions are not chemical reactions. Chemical reactions involve only the electrons of atoms; nuclear reactions involve the protons and neutrons in the atomic nuclei.
Four basic types[change | change source]
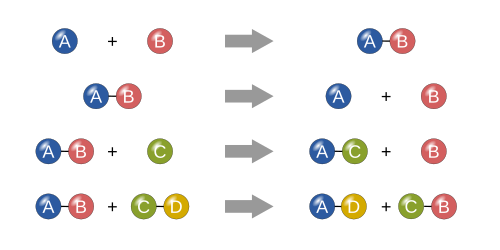
Synthesis[change | change source]
In a synthesis reaction, two or more simple substances combine to form a more complex substance.
"Two or more reactants giving one product" is another way to identify a synthesis reaction. One example of a synthesis reaction is the combination of iron and sulfur to form iron(II) sulfide:
Another example is simple hydrogen gas combined with simple oxygen gas to produce a more complex substance, such as water.[1]
Decomposition[change | change source]
A decomposition reaction is when a more complex substance breaks down into its more simple parts. It is thus the opposite of a synthesis reaction, and can be written as:[1][2]
One example of a decomposition reaction is the electrolysis of water to make oxygen and hydrogen gas:
Another example of a decomposition reaction is calcium carbonate breaking down into calcium oxide and carbon dioxide under high temperatures:
CaCO3 —> CaO + CO2
Single replacement[change | change source]
In a single replacement reaction, a single uncombined element replaces another in a compound; in other words, one element trades places with another element in a compound[1] These reactions come in the general form of:
One example of a single displacement reaction is when magnesium replaces hydrogen in water to make magnesium hydroxide and hydrogen gas:
Double replacement[change | change source]
In a double replacement reaction, the anions and cations of two compounds switch places and form two entirely different compounds.[1] These reactions are in the general form:[2]
For example, when barium chloride (BaCl2) and magnesium sulfate (MgSO4) react, the SO42− anion switches places with the 2Cl− anion, giving the compounds BaSO4 and MgCl2.
Another example of a double displacement reaction is the reaction of lead(II) nitrate with potassium iodide to form lead(II) iodide and potassium nitrate:
Equations[change | change source]
A chemical reaction is being displayed by an equation:
- Here, A and B react to C and D in a chemical reaction.
- This is an example of a combustion reaction.
Related pages[change | change source]
Other websites[change | change source]
- Rates of reaction Archived 2007-03-05 at the Wayback Machine
- Online chemical equation balancer Balances equation of any chemical reaction (full or half-cell) in one click.
References[change | change source]
- ↑ 1.0 1.1 1.2 1.3 To react or not to react? Archived 2015-01-10 at the Wayback Machine Utah State Office of Education. Retrieved 4 June 2011
- ↑ 2.0 2.1 Six types of chemical reactions – MrGuch ChemFiesta.










