Diffusion
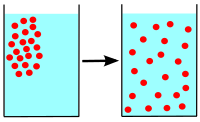
Diffusion is a process where molecules of a material move from an area of high concentration (where there are many molecules) to an area of low concentration (where there are fewer molecules)[1]until it has reached equilibrium (molecules evenly spread).
Diffusion usually happens in a mixture in gas, a liquid and occasionally colloids. It is possible to see diffusion happening when two liquids are mixed in a transparent container. It describes the constant movement of particles in all liquids, gases and colloids. These particles move in all directions bumping into each other.
Examples[change | change source]
- A sugar cube is left in a beaker of water for a while.
- The smell of ammonia spreads from the front of the classroom to the back of the room.
- Fumes of perfume rise from the bottle when the top is removed.
- Food coloring dropped on the beaker spreads out.
- the smell of food spread in the whole house
Molecules tend to move from places of high concentration to places of low concentration, just by moving randomly. For example, there is more oxygen in a lung than there is oxygen in the blood so oxygen molecules will tend to move into the blood. Similarly, there is more carbon dioxide molecules in the blood than in the lung so carbon dioxide molecules will tend to move into the lung. It happens in cell biology, where small molecules simply diffuse through the cell membrane, but larger molecules only get through by using energy: see active transport.
The random movement of fluid molecules makes them spread out until a boundary stops them.
Diffusion is a passive process, therefore does not require energy as it occurs down a concentration gradient.
Osmosis and heat transfer are types of diffusion.
Rate of Diffusion[change | change source]
Diffusion is affected by:
- the concentration gradient - diffusion will be greater where gradient is larger
- the temperatures - diffusion will happen faster when temperatures are higher as there is more kinetic energy
- the surface area - diffusion will be greater where it's greater
- the diffusion distance - diffusion will be greater where there is a short diffusion distance
Surface Area and Volume[change | change source]
In small unicellular organisms, simple diffusion can exchange molecules quickly enough to keep them alive. A high surface area to volume ratio helps.
However, for multicellular organism simple diffusion is not enough. They need to move more material over longer distances to remain alive. They have evolved to have internal structures and systems for rapid distribution movement. For example, humans have lungs to make diffusion happen rapidly. The same happens in plants with the leaf.
