Supramolecular chemistry
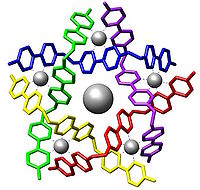
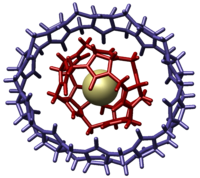




Supramolecular chemistry is area of chemistry that studies the relationship and linking molecules into bigger systems. It focuses on the chemical systems made up of a discrete number of assembled molecular subunits or components.[2][3][4] The study of non-covalent interactions is crucial to understanding many biological processes from cell structure to vision that rely on these forces for structure and function. Biological systems are often the inspiration for supramolecular research.
Related pages[change | change source]
References[change | change source]
- ↑ Hasenknopf, Bernold; Lehn, Jean-Marie; Kneisel, Boris O.; Baum, Gerhard; Fenske, Dieter (1996). "Self-Assembly of a Circular Double Helicate". Angewandte Chemie International Edition in English. 35 (16): 1838. doi:10.1002/anie.199618381.
- ↑ Lehn JM (1993). "Supramolecular chemistry". Science. 260 (5115): 1762–3. Bibcode:1993Sci...260.1762L. doi:10.1126/science.8511582. PMID 8511582.
- ↑ Supramolecular Chemistry, J.-M. Lehn, Wiley-VCH (1995) ISBN 978-3527293117
- ↑ Gennady V. Oshovsky, David N. Reinhoudt, Willem Verboom (2007). "Supramolecular Chemistry in Water" (PDF). Angewandte Chemie International Edition. 46 (14): 2366–2393. doi:10.1002/anie.200602815. PMID 17370285. S2CID 5969241.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Other websites[change | change source]
Wikimedia Commons has media related to Supramolecular chemistry.
- 2D and 3D Models of Dodecahedrane and Cuneane Assemlies [1] Archived 2011-05-22 at the Wayback Machine
- Supramolecular Chemistry - Thematic Series in the Open Access Beilstein Journal of Organic Chemistry
