User:Peaceray/Octamethylendiamine
Structural formula of octamethylenediamine
| |
| Names | |
|---|---|
| IUPAC name
Octane-1,8-diamine
| |
Other names
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| Abbreviations | OMDA |
| Beilstein Reference | 3-04-00-00612 |
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| |
| Properties | |
| C8H20N2 | |
| Molar mass | 144.26 g mol−1 |
| Appearance | colorless solid with an amine-like odor[1] |
| Density | 0.83 g cm−3 (60 °C)[1] |
| Melting point | 52 °C[3] |
| Boiling point | 225-226 °C[1][2] |
| Easily soluble in water (575 g l−1 at 20°C)[1] | |
| Vapor pressure | |
| Hazards | |
| Flash point | 113 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Octamethylenediamine (OMDA) is an organic chemical compound from the substance group of aliphatic diamines. It is used as a versatile reaction intermediate in the manufacture of pesticides, especially fungicides.
Manufacture
[change | change source]The industrial production of octamethylene diamine is carried out by the catalytic hydrogenation of suberonitrile at temperatures of 150 to 180 °C and a pressure of 50 to 180 bar in the presence of ammonia over heterogeneous cobalt unsupported catalysts:
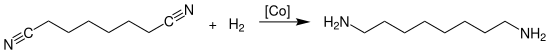
The reaction is carried out in the liquid phase and is carried out continuously or batchwise. The catalyst is arranged as a fixed bed in a shaft, tube, or tube bundle reactor.
Characteristics
[change | change source]Octamethylenediamine is a combustible but difficult to ignite. It is a solid that is easily soluble in water. The aqueous solutions are strongly alkaline (pH value of 12.1 at a concentration of 10 g/l).[4]
Use
[change | change source]Octamethylenediamine is used as a versatile intermediate in manufacturing pesticides, especially fungicides.[5]
Safety instructions
[change | change source]While octamethylenediamine is combustible, it is difficult to ignite because it is solid at moderate temperatures. It has a lower explosive limit (LEL) of 1.1 % by volume and an upper explosive limit (UEL) of 6.8 % by volume. The ignition temperature is 280 °C The substance therefore falls into temperature class T3. With a flash point of 113 °C, the liquid is considered difficult to ignite.[4]
References
[change | change source]- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Record of Octamethylenediamine in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 2022-01-20.
- ↑ "373-44-4 - 1,8-Diaminooctane, 98% - 1,8-Octanediamine - B23885". Alfa Aesar. 2017-10-22. Retrieved 2022-08-06.
- ↑ "1,8-Octanediamine". CAS Common Chemistry. Retrieved 2022-08-05.
- ↑ 4.0 4.1 "WO2018050555A1 Method for the Preparation of Polyamines from Dinitriles and/or Amino Nitrile". Espacenet – patent search. 2021-03-20. Retrieved 2022-08-05.
- ↑ Peter Roose, Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke: Amines, Aliphatic. In: Ullmann’s Encyclopedia of Industrial Chemistry. Wiley‐VCH Verlag GmbH & Co. KGaA., 30. September 2015, doi:10.1002/14356007.a02_001.pub2.
External links
[change | change source]- "1,8-diaminooctane". MetaCyc. 2022-08-05. Retrieved 2022-08-06.
- Thalladi, V.R.; Boese, R.; Weiss, H.-C. (2001), CCDC 132875: Experimental Crystal Structure Determination, Cambridge Crystallographic Data Centre, doi:10.5517/CC4G894, retrieved 2022-08-06
