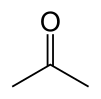
Acetone
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Propan-2-one[7] | |||
| Other names | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| 3DMet | |||
| Beilstein Reference | 635680 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.602 | ||
| EC Number |
| ||
| Gmelin Reference | 1466 | ||
| KEGG | |||
| MeSH | Acetone | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1090 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| C3H6O | |||
| Molar mass | 58.08 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | Pungent, irritating, floral, cucumber like | ||
| Density | 0.7845 g/cm3 (25 °C) | ||
| Melting point | −94.7 °C (−138.5 °F; 178.5 K)[12] | ||
| Boiling point | 56.05 °C (132.89 °F; 329.20 K)[12] | ||
| Miscible | |||
| Solubility | Miscible in benzene, diethyl ether, methanol, chloroform, ethanol[8] | ||
| log P | -0.16[9] | ||
| Vapor pressure |
| ||
| Acidity (pKa) | |||
| −33.78·10−6 cm3/mol | |||
Refractive index (nD)
|
1.3588 (VD = 54.46) | ||
| Viscosity | 0.295 mPa·s (25 °C)[8] | ||
| Structure | |||
| Trigonal planar at C2 | |||
| Dihedral at C2 | |||
| 2.91 D | |||
| Thermochemistry | |||
| Std enthalpy of formation ΔfH |
(−250.03) – (−248.77) kJ/mol | ||
| Std enthalpy of combustion ΔcH |
−1.772 MJ/mol | ||
| Standard molar entropy S |
200.4 J/(mol·K) | ||
| Specific heat capacity, C | 125.45 J/(mol·K) | ||
| Hazards | |||
| NFPA 704 |
| ||
| Explosive limits | 2.6–12.8%[13] | ||
| U.S. Permissible exposure limit (PEL) |
1000 ppm (2400 mg/m3)[6] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Acetone, or propanone, is an organic compound with the chemical formula (CH3)2CO. This clear, mobile, easy-to-burn liquid is the simplest example of the ketones. Acetone can be mixed with water. It is an important solvent, often to clean things in the laboratory. Common uses of acetone in the home are as the active ingredient in nail polish remover and as paint thinner. It is a common building block in organic chemistry.
Sources[change | change source]
- ↑ The Merck Index, 15th Ed. (2013), p. 13, Acetone Monograph 65, O'Neil: The Royal Society of Chemistry.(subscription required)
- ↑ 2.0 2.1 2.2 2.3 Acetone in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD), http://webbook.nist.gov (retrieved 2014-05-11)
- ↑ Klamt, Andreas (2005). COSMO-RS: From Quantum Chemistry to Fluid Phase Thermodynamics and Drug Design. Elsevier. pp. 92–94. ISBN 978-0-444-51994-8.
- ↑ Ash, Michael; Ash, Irene (2004). Handbook of preservatives. Synapse Information Resources, Inc. p. 369. ISBN 1-890595-66-7.
- ↑ Myers, Richard L. (2007). The 100 Most Important Chemical Compounds: A Reference Guide. Greenwood. pp. 4–6. ISBN 978-0-313-08057-9.
- ↑ 6.0 6.1 NIOSH Pocket Guide to Chemical Hazards. "#0260". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 723. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ 8.0 8.1 Properties of substance: acetone. chemister.ru.
- ↑ "acetone". ChemSrc. Retrieved 2018-04-13.
- ↑ Chiang, Yvonne; Kresge, A. Jerry; Tang, Yui S.; Wirz, Jakob (1984). "The pKa and keto-enol equilibrium constant of acetone in aqueous solution". Journal of the American Chemical Society. 106 (2): 460–462. doi:10.1021/ja00314a055.
- ↑ Bordwell, Frederick G. (1988). "Equilibrium acidities in dimethyl sulfoxide solution". Accounts of Chemical Research. 21 (12): 456–463. doi:10.1021/ar00156a004. S2CID 26624076.
- ↑ 12.0 12.1 Lide, David R. (ed) (2003). CRC Handbook of Chemistry and Physics, 84th Edition. CRC Press. Boca Raton, Florida; Section 3, Physical Constants of Organic Compounds.
- ↑ "Working with modern hydrocarbon and oxygenated solvents: a guide to flammability". American Chemistry Council Solvents Industry Group. January 2008. p. 7. Archived from the original on 2009-06-01.