Trinitrotoluene
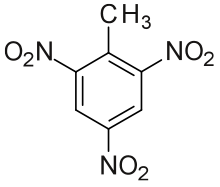
Trinitrotoluene (Abbreviated TNT) (/ˌtraɪˌnaɪtroʊˈtɒljuiːn/) more specifically 2,4,6-trinitrotoluene, is a powerful nitroaromatic explosive, occasionally used as a reagent in chemical synthesis.
History[change | change source]
TNT was first made in 1863 by Julius Wilbrand[1] and originally used as a yellow dye. It was not recognized as an explosive for 28 years, until the chemist Carl Häussermann discovered its explosive properties in 1891.[2]

The German military started filling artillery shells with TNT 1902. TNT-filled shells would explode after they had broken through the armour of ships, whereas the British Lyddite (Picric acid)-filled shells exploded when they hit the armour, wasting energy outside the ship.[3] The British started replacing Pciric acid with TNT in 1907.[4]
Uses[change | change source]
TNT is one of the world's most commonly used explosives, in the military, mining, and industrial operations.
TNT is often blended with other explosives to get desired properties.[source?]
References[change | change source]
- ↑ Annalen der Pharmacie (in German). Winter. 1863.
- ↑ Krehl, Peter O. K. (2008-09-24). History of Shock Waves, Explosions and Impact: A Chronological and Biographical Reference. Springer Science & Business Media. ISBN 978-3-540-30421-0.
- ↑ Brown, G. I. (1998). The big bang. Internet Archive. Sutton Pub. ISBN 978-0-7509-1878-7.
- ↑ Skentelbery, Norman (1975). Arrows to Atom Bombs: A History of the Ordnance Board. Ordnance Board.
