Electron
An electron is a very small piece of matter. Its symbol is e−, and it was discovered by J. J. Thomson in 1897.
The electron is a subatomic particle. Every atom is made of some electrons that surround the nucleus of the atom. An electron can also be separate from any atom. It is believed to be an elementary particle because it cannot be broken down into anything smaller.[1] Its electric charge is negative.[2] Electrons have very little mass (little weight) so very little energy is needed to move them fast. They may move almost at the speed of light, for instance, as beta particles, and in the inner electron shells of elements with a large atomic number. [3]
Electrons take part in gravitational, electromagnetic and weak interactions.[4] The electromagnetic force is strongest in common situations. Electrons repel (push apart) from each other because they have the same electric charge. Electrons are attracted to protons because they have opposite electric charge. An electron has an electric field, which describes these forces. The electricity that powers televisions, motors, mobile phones, basically everything (electric) is actually many electrons moving through wires or other conductors.
Description[change | change source]
Electrons have the smallest electrical charge. This electrical charge equals the charge of a proton, but has the opposite sign. For this reason, electrons are attracted towards the protons in atomic nuclei. This attraction makes electrons near a nucleus form an atom. An electron has a mass of about 1/1836 times a proton.[5]
One way to think about the location of electrons in an atom is to imagine that they orbit at fixed distances from the nucleus. This way, electrons in an atom exist in a number of electron shells surrounding the central nucleus. Each electron shell is given a number 1, 2, 3, and so on, starting from the one closest to the nucleus (the innermost shell). Each shell can hold up to a certain maximum number of electrons. The distribution of electrons in the various shells is called electronic arrangement (or electronic form or shape). Electronic arrangement can be shown by numbering or an electron diagram. (A different way to think about the location of electrons is to use quantum mechanics to calculate their atomic orbitals.)
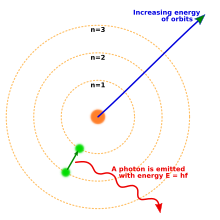
The electron is one of a type of subatomic particles called leptons. The electron has a negative electric charge. The electron has another property, called spin. Its spin value is 1/2, which makes it a fermion.
While most electrons are found in atoms, others move independently in matter, or together as cathode rays in a vacuum. In some superconductors, electrons move in pairs. When electrons flow, this flow is called electricity, or an electric current.
An object can be described as 'negatively charged' if there are more electrons than protons in an object, or 'positively charged' when there are more protons than electrons. Electrons can move from one object to another when touched. They may be attracted to another object with opposite charge, or repelled when they both have the same charge. When an object is 'grounded', electrons from the charged object go into the ground, making the object neutral. This is what lightning rods (lightning conductors) do.
Chemical reactions[change | change source]
Electrons in their shells round an atom are the basis of chemical reactions. Complete outer shells, with maximum electrons, are less reactive. Outer shells with less than maximum electrons are reactive. The number of electrons in atoms is the underlying basis of the chemical periodic table.[6]
Measurement[change | change source]
Electric charge can be directly measured with a device called an electrometer. Electric current can be directly measured with a galvanometer. The measurement given off by a galvanometer is different from the measurement given off by an electrometer. Today laboratory instruments are capable of containing and observing individual electrons.
'Seeing' an electron[change | change source]
In laboratory conditions, the interactions of individual electrons can be observed by means of particle detectors, which allow measurement of specific properties such as energy, spin and charge.[7] In one instance a Penning trap was used to contain a single electron for 10 months.[8] The magnetic moment of the electron was measured to a precision of eleven digits, which, in 1980, was a greater accuracy than for any other physical constant.[9]
The first video images of an electron's energy distribution were captured by a team at Lund University in Sweden, February 2008. The scientists used extremely short flashes of light, called attosecond pulses, which allowed an electron's motion to be observed for the first time.[10][11] The distribution of the electrons in solid materials can also be visualized.[12]
Anti-particle[change | change source]
The antiparticle of the electron is called a positron. This is identical to the electron, but carries electrical and other charges of the opposite sign. When an electron collides with a positron, they may scatter off each other or be totally annihilated, producing a pair (or more) of gamma ray photons.
History of its discovery[change | change source]
The effects of electrons were known long before it could be explained. The Ancient Greeks knew that rubbing amber against fur attracted small objects. Now we know the rubbing strips off electrons, and that gives an electric charge to the amber. Many physicists worked on the electron. J.J. Thomson proved it existed,[13] in 1897, but another man gave it the name 'electron'.[14]
The electron cloud model[change | change source]
The model views electrons as holding indeterminate positions in a diffuse cloud around the nucleus of the atom.
The uncertainty principle means a person cannot know an electron's position and energy level at the same time. These potential states form a cloud around the atom. The potential states of electrons in a single atom form a single, uniform cloud.
Related pages[change | change source]
References[change | change source]
- ↑ Purcell, Edward M. 1985. Electricity and Magnetism. Berkeley Physics Course Volume 2. McGraw-Hill. ISBN 0-07-004908-4.
- ↑ "JERRY COFF". Retrieved 10 September 2010.
- ↑ US Dept. of Energy: [1] Archived 2010-05-30 at the Wayback Machine
- ↑ Anastopoulos, Charis 2008. Particle or Wave: the evolution of the concept of matter in modern physics. Princeton University Press. pp261–262. ISBN 0691135126. https://books.google.com/books?id=rDEvQZhpltEC&pg=PA261
- ↑ "CODATA value: proton-electron mass ratio". 2006 CODATA recommended values. National Institute of Standards and Technology. Retrieved 2009-07-18.
- ↑ Pauling, Linus C. 1960. The nature of the chemical bond and the structure of molecules and crystals: an introduction to modern structural chemistry (3rd ed). Cornell University Press. pp4–10. ISBN 0801403332. https://books.google.com/books?id=L-1K9HmKmUUC
- ↑ Grupen, Claus 1999. "Physics of Particle Detection". AIP Conference Proceedings, Instrumentation in Elementary Particle Physics, VIII. 536. Istanbul: Dordrecht, D. Reidel Publishing Company. pp. 3–34. doi:10.1063/1.1361756.
- ↑ Staff (2008). "The Nobel Prize in Physics 1989". The Nobel Foundation. Retrieved 2008-09-24.
- ↑ Ekstrom, Philip (1980). "The isolated Electron" (PDF). Scientific American. 243 (2): 91–101. doi:10.1038/scientificamerican0880-104. Retrieved 2008-09-24.
- ↑ Mauritsson, Johan. "Electron filmed for the first time ever" (PDF). Lunds Universitet. Archived from the original (PDF) on 2009-03-25. Retrieved 2008-09-17.
- ↑ Mauritsson, J.; Johnsson, P.; Mansten, E.; Swoboda, M.; Ruchon, T.; L’huillier, A.; Schafer, K. J. (2008). "Coherent Electron Scattering Captured by an Attosecond Quantum Stroboscope". Physical Review Letters. 100 (7): 073003. doi:10.1103/PhysRevLett.100.073003. PMID 18352546. S2CID 1357534. Archived from the original (PDF) on 2017-02-27. Retrieved 2010-07-16.
- ↑ Damascelli, Andrea (2004). "Probing the electronic structure of complex systems by ARPES". Physica Scripta. T109: 61–74. arXiv:cond-mat/0307085. doi:10.1238/Physica.Topical.109a00061. S2CID 21730523.
- ↑ Davis & Falconer, J.J. Thomson and the Discovery of the Electron
- ↑ Shipley, Joseph T. 1945. Dictionary of word origins. The Philosophical Library. p133.
Other websites[change | change source]
- "The Discovery of the Electron". American Institute of Physics, Center for History of Physics. Archived from the original on 2008-03-16. Retrieved 2012-11-26.
- "Particle Data Group". University of California.
- Bock, R.K.; Vasilescu, A. (1998). The Particle Detector BriefBook (14th ed.). Springer. ISBN 3-540-64120-3.
