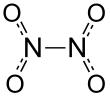
Dinitrogen tetroxide
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Dinitrogen tetraoxide
| |||
| Other names
Dinitrogen(II) oxide(-I)
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.031.012 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UN number | 1067 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| N2O4 | |||
| Molar mass | 92.011 g/mol | ||
| Appearance | colourless liquid / orange gas | ||
| Density | 1.44246 g/cm3 (liquid, 21 °C) | ||
| Melting point | −11.2 °C (11.8 °F; 261.9 K) | ||
| Boiling point | 21.69 °C (71.04 °F; 294.84 K) | ||
| reacts | |||
| Vapor pressure | 96 kPa (20 °C)[1] | ||
Refractive index (nD)
|
1.00112 | ||
| Structure | |||
| planar, D2h | |||
| zero | |||
| Thermochemistry | |||
| Std enthalpy of formation ΔfH |
+9.16 kJ/mol[2] | ||
| Standard molar entropy S |
304.29 J K−1 mol−1[2] | ||
| Hazards | |||
| MSDS | External MSDS | ||
| EU classification | Very toxic (T+) Corrosive (C) | ||
| EU Index | 007-002-00-0 | ||
| NFPA 704 |
| ||
| R-phrases | R26, R34 | ||
| S-phrases | (S1/2), S9, S26, S28, S36/37/39, S45 | ||
| Flash point | Non-flammable | ||
| Related compounds | |||
| Related nitrogen oxides | Nitrous oxide Nitric oxide Dinitrogen trioxide Nitrogen dioxide Dinitrogen pentoxide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Dinitrogen tetroxide, also known as nitrogen tetroxide or dinitrogen tetraoxide, is a chemical compound. Its chemical formula is N2O4. It contains nitrogen in its +4 oxidation state. It contains nitrogen and oxide ions.
Properties[change | change source]
It is a colorless gas, although it is sometimes polluted with nitrogen dioxide. It is very corrosive and a strong oxidizing agent. It can ignite on contact with hydrazine. It can be made by bonding two nitrogen dioxide molecules together at a low temperature or a high pressure.
Preparation[change | change source]
It is made by bonding nitrogen dioxide molecules together in pairs.
Uses[change | change source]
It is used as a rocket propellant, along with hydrazine. This mixture is good since it does not have to be ignited. It is used similar to nitrogen dioxide to make nitric acid. It can react with metals to make nitrates.
Safety[change | change source]
Dinitrogen is highly toxic and corrosive. Some astronauts breathed it and had to go to a hospital.
Related pages[change | change source]
Sources[change | change source]
- ↑ International Chemical Safety Card
- ↑ 2.0 2.1 P.W. Atkins and J. de Paula, Physical Chemistry (8th ed., W.H. Freeman, 2006) p.999