User:Mr. Ibrahem/Levonorgestrel
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Plan B, others |
| Synonyms | LNG; d-Norgestrel; d(–)-Norgestrel; D-Norgestrel; WY-5104; SH-90999; NSC-744007; 18-Methylnorethisterone; 17α-Ethynyl-18-methyl-19-nortestosterone; 17α-Ethynyl-18-methylestr-4-en-17β-ol-3-one; 13β-Ethyl-17α-hydroxy-18,19-dinorpregn-4-en-20-yn-3-one |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a610021 |
| Pregnancy category | |
| Routes of administration | By mouth, transdermal patch, intrauterine device, subcutaneous implant |
| Drug class | Progestogen; progestin |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 95% (range 85–100%) |
| Protein binding | 98% (50% to albumin, 48% to SHBG) |
| Metabolism | Liver (reduction, hydroxylation, conjugation) |
| Metabolites | • 5α-Dihydro-@@@6@@@ |
| Elimination half-life | 24–32 hours |
| Excretion | Urine: 20–67% Feces: 21–34% |
| Identifiers | |
| |
| Chemical and physical data | |
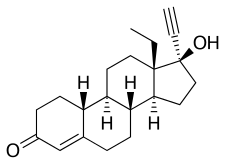
| Formula | C21H28O2 |
| Molar mass | 312.45 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 235 to 237 °C (455 to 459 °F) |
| |
| |
| (verify) | |
Levonorgestrel is a hormonal medication which is used in a number of birth control methods.[2] It is combined with an estrogen to make combination birth control pills.[3] As an emergency birth control, sold under the brand name Plan B among others, it is useful within 120 hours of unprotected sex.[2] The more time that has passed since sex, the less effective the medication becomes, and it does not work after pregnancy (implantation) has occurred.[2] It decreases the chances of pregnancy by 57 to 93%.[4] In an intrauterine device (IUD), such as Mirena among others, it is effective for the long-term prevention of pregnancy.[2] A levonorgestrel-releasing implant is also available in some countries.[5]
Common side effects include nausea, breast tenderness, headaches, and increased, decreased, or irregular menstrual bleeding.[2] When used as an emergency contraceptive, if pregnancy occurs, there is no evidence that its use harms the baby.[2] It is safe to use during breastfeeding.[2] Birth control that contains levonorgestrel will not change the risk of sexually transmitted infections.[2] It is a progestin and has effects similar to those of the hormone progesterone.[2] It works primarily by preventing ovulation and closing off the cervix to prevent the passage of sperm.[2]
Levonorgestrel was patented in 1960 and introduced for medical use together with ethinylestradiol in 1970.[6] It is on the World Health Organization's List of Essential Medicines.[7] It is available as a generic medication.[8] The wholesale cost in the developing world is between $0.23 and $1.65 US for the dose required for emergency birth control.[9] In the United States, levonorgestrel-containing emergency birth control is available over the counter (OTC) for all ages.[10] In 2016, it was the 223rd most commonly prescribed medication in the United States, with more than two million prescriptions.[11]
References
[change | change source]- ↑ 1.0 1.1 1.2 "Levonorgestrel Use During Pregnancy". Drugs.com. 23 March 2020. Archived from the original on 2 July 2020. Retrieved 29 June 2020.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 "Progestins (Etonogestrel, Levonorgestrel, Norethindrone)". The American Society of Health-System Pharmacists. Archived from the original on 2015-09-07. Retrieved Aug 21, 2015.
- ↑ Postgraduate Gynecology. Jaypee Brothers Medical Pub. 2011. p. 159. ISBN 9789350250822. Archived from the original on 2015-09-26.
- ↑ Gemzell-Danielsson, K (November 2010). "Mechanism of action of emergency contraception". Contraception. 82 (5): 404–9. doi:10.1016/j.contraception.2010.05.004. PMID 20933113.
- ↑ "Chapter 1". Research on reproductive health at WHO : biennial report 2000-2001. Geneva: World health organization. 2002. ISBN 9789241562089. Archived from the original on 2015-09-26.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 479. ISBN 9783527607495. Archived from the original on 2021-08-28. Retrieved 2020-08-05.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ Hamilton, Richard J. (2014). Tarascon pocket pharmacopoeia : 2014 deluxe lab-pocket edition (15th ed.). Sudbury: Jones & Bartlett Learning. pp. 310–312. ISBN 9781284053999. Archived from the original on 2015-09-26.
- ↑ "Levonorgestrel". International Drug Price Indicator Guide. Archived from the original on 22 January 2018. Retrieved 21 August 2015.
- ↑ "FDA approves Plan B One-Step emergency contraceptive for use without a prescription for all women of child-bearing potential" (Press release). June 20, 2013. Archived from the original on 14 January 2016. Retrieved 2 February 2016.
- ↑ "Levonorgestrel - Drug Usage Statistics". ClinCalc. Archived from the original on 2 July 2020. Retrieved 11 April 2020.
