Aldehyde
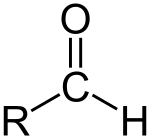

An aldehyde (/ˈældɪhaɪd/) is an organic compound. It contains a formyl group. A formyl group is a part of a molecule with the structure R-CHO. It is made of a carbon double bonded to oxygen. The carbon is also bonded to hydrogen and an R group.[1] A side chain is the rest of the molecule. The group without the side chain is called the aldehyde group or formyl group. Aldehydes are different from ketones because the formyl group is at the end of the molecule in an aldehyde. Ketones have the formyl group in the middle of the molecule. Aldehydes are common in organic chemistry. Many fragrances (smell producing compounds) are aldehydes.
Structure and bonding
[change | change source]Aldehydes have a carbon double bonded to an oxygen. Because of this, they are slightly polar. This causes aldehydes to have a number of properties. One of the most important of these is solubility in water.
Nomenclature
[change | change source]IUPAC names for aldehydes
[change | change source]The common names for aldehydes are not always the official names. The IUPAC names are useful, however. IUPAC recommends the following nomenclature for aldehydes:[2][3][4]
- Aldehydes without rings of carbon are named based on the longest carbon chain containing the aldehyde. Thus, HCHO is named based on methane, and CH3CH2CH2CHO is named based on butane. The name is formed by changing the ending -e of the parent alkane to -al. HCHO is named methanal. CH3CH2CH2CHO is named butanal.
- If a -CHO group is attached to a ring, then -carbaldehyde is added to the end of the name. Because of this, C6H11CHO is known as cyclohexanecarbaldehyde. If there are other names that need to be added to the end of the word because other functional groups are present, formyl- is added to the beginning of the word. Methanoyl-is sometimes added instead, but formyl- is better.
Etymology
[change | change source]
The word aldehyde comes from Latin. It was shortened from alcohol dehydrogenatus (dehydrogenated alcohol). The word aldehyde was created by Justus von Liebig.[5] In the past, aldehydes were sometimes named after the corresponding alcohols, for example, vinous aldehyde for acetaldehyde. (Vinous is from the Latin word for wine, which is the traditional source of ethanol.)
The term formyl group comes from the Latin or Italian word formica which means ant.
Physical properties and characterization
[change | change source]Aldehydes have many different properties. These properties change a lot if the rest of the molecule changes. Smaller aldehydes are more soluble in water. Formaldehyde and acetaldehyde are completely soluble in water. Many aldehydes have strong smells. Aldehydes will break down in air.
Two of the most important aldehydes, formaldehyde and acetaldehyde, tend to form long chains. This is called polymerization.
Naturally occurring aldehydes
[change | change source]Many aldehydes are found in essential oils and are the reason for their smells. Cinnamaldehyde, cilantro, and vanillin all get their smells from aldehydes.
Common reactions
[change | change source]Aldehydes are highly reactive and participate in many reactions.[6]" In industry, aldehydes are used to prepare plasticizers, polyols, and alcohols. In biology, aldehydes are used to replace amine groups and form sugars.[6]
Dialdehydes
[change | change source]A dialdehyde is a molecule with two aldehydes. The names of dialdehydes have the ending -dial or -dialdehyde. Some dialdehydes are named after the acid that they are similar to. An example is butanedial, which is also called succinaldehyde (from succinic acid).
Examples of aldehydes
[change | change source]- Methanal (formaldehyde)
- Ethanal (acetaldehyde)
- Propanal (propionaldehyde)
- Butanal (butyraldehyde)
- Benzaldehyde
- Cinnamaldehyde
- Tolualdehyde
- Furfural
- Retinaldehyde
Dialdehydes
[change | change source]Uses
[change | change source]Of all aldehydes, formaldehyde is produced the most. About 6 million tons per year are made. It is mainly used to make resins when mixed with urea, melamine, and phenol. Bakelite is made like this. Another commonly made aldehyde is butyraldehyde. 2 and a half million tons per year are made. A lot of acetaldehyde once was once made, but much less is made today. This is because chemicals that used to be made from acetaldehyde are now made in other ways.
Related pages
[change | change source]References
[change | change source]- ↑ IUPAC Gold Book aldehydes
- ↑ Short Summary of IUPAC Nomenclature of Organic Compounds, web page, University of Wisconsin Colleges, accessed on line August 4, 2007.
- ↑ §R-5.6.1, Aldehydes, thioaldehydes, and their analogues, A Guide to IUPAC Nomenclature of Organic Compounds: recommendations 1993, IUPAC, Commission on Nomenclature of Organic Chemistry, Blackwell Scientific, 1993.
- ↑ §R-5.7.1, Carboxylic acids, A Guide to IUPAC Nomenclature of Organic Compounds: recommendations 1993, IUPAC, Commission on Nomenclature of Organic Chemistry, Blackwell Scientific, 1993.
- ↑ Crosland, Maurice P. (2004), Historical Studies in the Language of Chemistry, Courier Dover Publications, ISBN 9780486438023
- ↑ 6.0 6.1 Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 0-471-72091-7
Other websites
[change | change source]Entrancei https://www.entrancei.com/chapter-aldehydes-and-ketones/type-of-chemical-reactions-in-carbonyl-compounds Archived 2018-10-22 at the Wayback Machine
- Aldehyde synthesis - Synthetic protocols Archived 2015-09-26 at the Wayback Machine from organic-reaction.com
