Actinium
 | ||||||||||||||||||||||||||||||||||
| Actinium | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ækˈtɪniəm/ | |||||||||||||||||||||||||||||||||
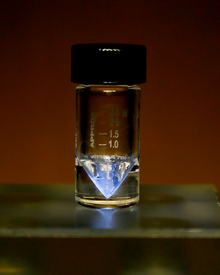
| Appearance | silvery-white, glowing with an eerie blue light;[1] sometimes with a golden cast[2] | |||||||||||||||||||||||||||||||||
| Mass number | [227] | |||||||||||||||||||||||||||||||||
| Actinium in the periodic table | ||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||
| Group | f-block groups (no number) | |||||||||||||||||||||||||||||||||
| Period | period 7 | |||||||||||||||||||||||||||||||||
| Block | f-block | |||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 6d1 7s2 | |||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 18, 9, 2 | |||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||||||||||
| Melting point | 1500 K (1227 °C, 2240 °F) (estimated)[2] | |||||||||||||||||||||||||||||||||
| Boiling point | 3500±300 K (3200±300 °C, 5800±500 °F) (extrapolated)[2] | |||||||||||||||||||||||||||||||||
| Density (near r.t.) | 10 g/cm3 | |||||||||||||||||||||||||||||||||
| Heat of fusion | 14 kJ/mol | |||||||||||||||||||||||||||||||||
| Heat of vaporization | 400 kJ/mol | |||||||||||||||||||||||||||||||||
| Molar heat capacity | 27.2 J/(mol·K) | |||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||
| Oxidation states | +3 (a strongly basic oxide) | |||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.1 | |||||||||||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||||||||||
| Covalent radius | 215 pm | |||||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||||||
| Natural occurrence | from decay | |||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic (fcc) | |||||||||||||||||||||||||||||||||
| Thermal conductivity | 12 W/(m⋅K) | |||||||||||||||||||||||||||||||||
| CAS Number | 7440-34-8 | |||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||
| Discovery and first isolation | Friedrich Oskar Giesel (1902) | |||||||||||||||||||||||||||||||||
| Named by | André-Louis Debierne (1899) | |||||||||||||||||||||||||||||||||
| Isotopes of actinium | ||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||
Actinium is chemical element 89 on the periodic table. Its symbol is Ac. Actinium's mass is 227 g/mol.
Actinium is a silver radioactive, solid metal in actinide group. It is so radioactive that it glows in the dark. Even a small amount of actinium is dangerous to people.
History[change | change source]
Actinium was discovered in 1899 by André-Louis Debierne, a French chemist. In 1899, Debierne described the substance as similar to titanium[4] and (in 1900) as similar to thorium.[5]
Properties[change | change source]
Actinium is a soft, silvery-white, radioactive metal.[6][7] Its estimated shear modulus is similar to that of lead. Because its strong radioactivity, actinium glows in the dark with a pale blue light.[8]
Actinium reacts quickly with oxygen and moisture in air forming a white coating of actinium oxide that stops the actinium from oxidizing.[6]
Isotopes[change | change source]
Actinium that is found in nature is made up of two radionuclides 227Ac and 228Ac. Thirty-six radionuclides of actinium have been found. The most stable is 227Ac which has a half-life of 21.772 years. The shortest-lived known isotope of actinium is 217Ac which has a half-life of 69 nanoseconds.
Occurence[change | change source]
Actinium is only found in trace amounts in uranium ores. For example, one tonne of uranium ore contains about 0.2 milligrams of 227Ac. Thorium ores contain about 5 nanograms of 228Ac per one tonne of thorium.[9]
Uses[change | change source]
225Ac is now being studied for use in cancer treatments. 227Ac is studied for use as an active element of radioisotope thermoelectric generators. 225Ac is used in medicine to make 213Bi in a reusable generator. It can be used in radiation therapy.[10][11][12]
References[change | change source]
- ↑ Wall, Greg (8 September 2003). "C&EN: It's Elemental: The Periodic Table - Actinium". C&EN: It's Elemental: The Periodic Table. Chemical and Engineering News. Retrieved 2 June 2011.
- ↑ 2.0 2.1 2.2 Kirby, Harold W.; Morss, Lester R. (2006). "Actinium". The Chemistry of the Actinide and Transactinide Elements. p. 18. doi:10.1007/1-4020-3598-5_2. ISBN 978-1-4020-3555-5.
- ↑ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ↑ Debierne, André-Louis (1899). "Sur un nouvelle matière radio-active". Comptes rendus (in French). 129: 593–595.
- ↑ Debierne, André-Louis (1900–1901). "Sur un nouvelle matière radio-actif – l'actinium". Comptes rendus (in French). 130: 906–908.
- ↑ 6.0 6.1 Stites, Joseph G.; Salutsky, Murrell L.; Stone, Bob D. (1955). "Preparation of Actinium Metal 2". Journal of the American Chemical Society. 77 (1): 237–240. doi:10.1021/ja01606a085. ISSN 0002-7863.
- ↑ Solid state physics : advances in research and applications. Volume 16. Seitz, Frederick, 1911-2008., Turnbull, David, 1915-2007. New York: Academic Press. 1964. ISBN 978-0-08-086480-8. OCLC 646775097.
{{cite book}}: CS1 maint: others (link) - ↑ Katz, J J; Manning, W M (1952). "Chemistry of the Actinide Elements". Annual Review of Nuclear Science. 1 (1): 245–262. Bibcode:1952ARNPS...1..245K. doi:10.1146/annurev.ns.01.120152.001333. ISSN 0066-4243. Archived from the original on 2020-07-30. Retrieved 2020-10-15.
- ↑ Hagemann, French (1950). "The Isolation of Actinium 1". Journal of the American Chemical Society. 72 (2): 768–771. doi:10.1021/ja01158a033. ISSN 0002-7863.
- ↑ CRC handbook of chemistry and physics. Lide, David R., 1928- (86th ed., 2005-2006 ed.). Boca Raton: CRC Press. 2005. ISBN 0-8493-0486-5. OCLC 61108810.
{{cite book}}: CS1 maint: others (link) - ↑ Deblonde, Gauthier J.-P.; Abergel, Rebecca J. (2016). "Active actinium". Nature Chemistry. 8 (11): 1084. Bibcode:2016NatCh...8.1084D. doi:10.1038/nchem.2653. ISSN 1755-4330. PMID 27768109. S2CID 5404889.
- ↑ Boll, Rose A.; Malkemus, Dairin; Mirzadeh, Saed (2005). "Production of actinium-225 for alpha particle mediated radioimmunotherapy". Applied Radiation and Isotopes. 62 (5): 667–679. doi:10.1016/j.apradiso.2004.12.003. PMID 15763472.
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | ||||||||||
| |||||||||||||||||||||||||||||||||||||||||


